
alkali, and alkaline-earth metals, tend to be good reducing agents, as their valence electrons, whose radial orbit DEFINES the atomic radius, tend to be readily oxidized. On the other hand, the larger elements, i.e. nitrogen, fluorine, oxygen, TEND to be very powerful oxidants, and this is also manifested by their small atomic size. This is because their ionisation energies decrease as you go down. Excluding the Noble Gases, the smaller atoms from the right hand side, i.e. In fact, the overall reactivity of the alkaline metals increases as you move down the group.
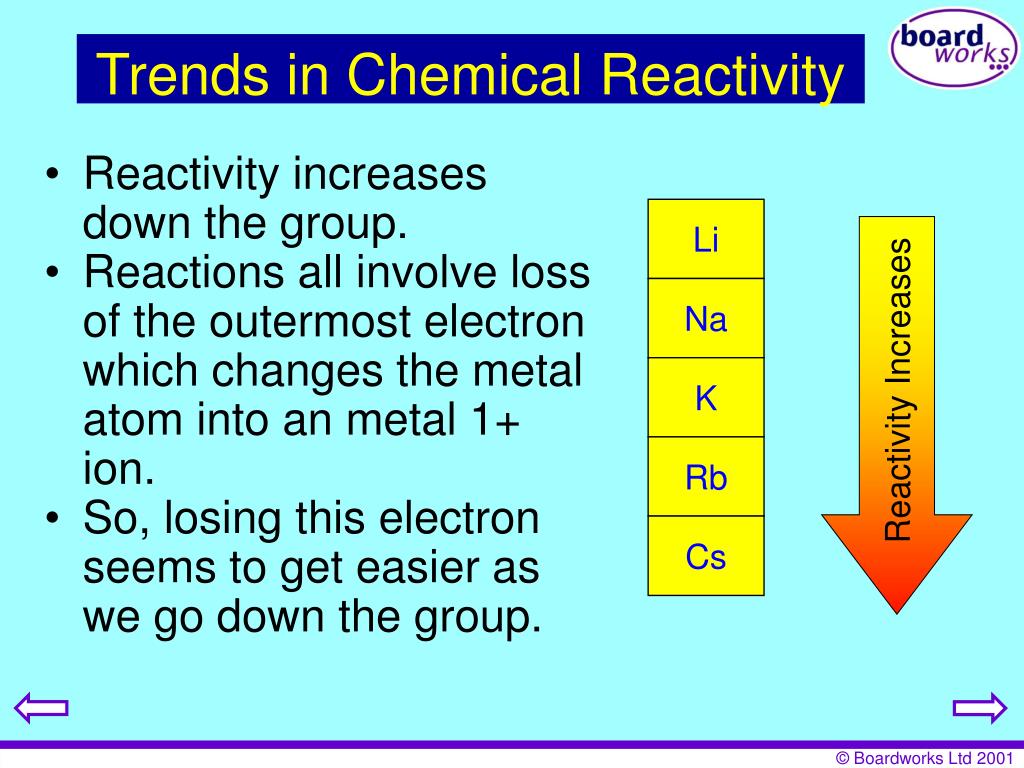
Therefore, as you go down the group the outermost electron becomes easier to lose. Group - reactivity decreases as you go down the group. Group - reactivity increases as you go down a group In Non-metals Period - reactivity increases as you go from the left to the right. It follows that the SMALLEST atoms derive the right of the Table as we face it. As you go down the group the reactivity of the Group 1 increases because. In Metals: Period - reactivity decreases as you go from left to right. That is why as you go up a group Chemical Reactivity increases because it is easier for elements to gain electrons when they have high electronegativity. Of course, the diagram shows NO data (it should do so), but the relative size of the atoms across the Period, and down the Group is clear. And the best metric that illustrates this trend is the well-known diminution of atomic radii across the Period from left to right? And of course, we should look at some data. Now it is a fact that the nuclear charge is SHIELDED very poorly by incomplete electronic shells. The chemistry and atomic structure of the elements is a contest between (i) nuclear charge, conveniently represented by #Z_"the atomic number"#, and (ii) shielding by other electrons. Metal reactivity relates to ability to lose electrons (oxidize), form basic hydroxides, form ionic compounds with non-metals.

#"Increase in atomic radii down a Group, a column of the Periodic"#"Table."#


 0 kommentar(er)
0 kommentar(er)
